A couple of weeks ago, a team of five jellyfish and polyp enthusiasts travelled to Torsvåg, Vannøya, a beautiful location two hours north of Tromsø by car (more about the fieldwork can be found here). Our goal? Collect, identify and catalogue some little-known hydrozoans for Artsdatabanken project NOAH (Norwegian Arctic Hydrozoa). This is the 3rd dedicated NOAH trip to obtain Arctic samples (or examine collections) in the last 12 months! After a very successful Arctic expedition to the West and North of Svalbard on board Kronprins Haakon in the context of the Barents Sea Ecosystem Survey by IMR, and a 1-week workshop at the Institute of Oceanology Polish Academy of Sciences (IOPAN) co-organized by our colleague Marta Ronowicz, this time we focused on the underexplored coasts of northern Norway.

Recent NOAH-related sampling trips. On top, Barents Sea Ecosystem Survey by IMR on board icebreaker Kronprins Haakon; in the middle, at IOPAN examining Marta Ronowicz’s extensive collection; bottom, NOAH team in Torsvåg (Troms). Pics: Joan Soto, Piotr Bałazy, Robert Johansen
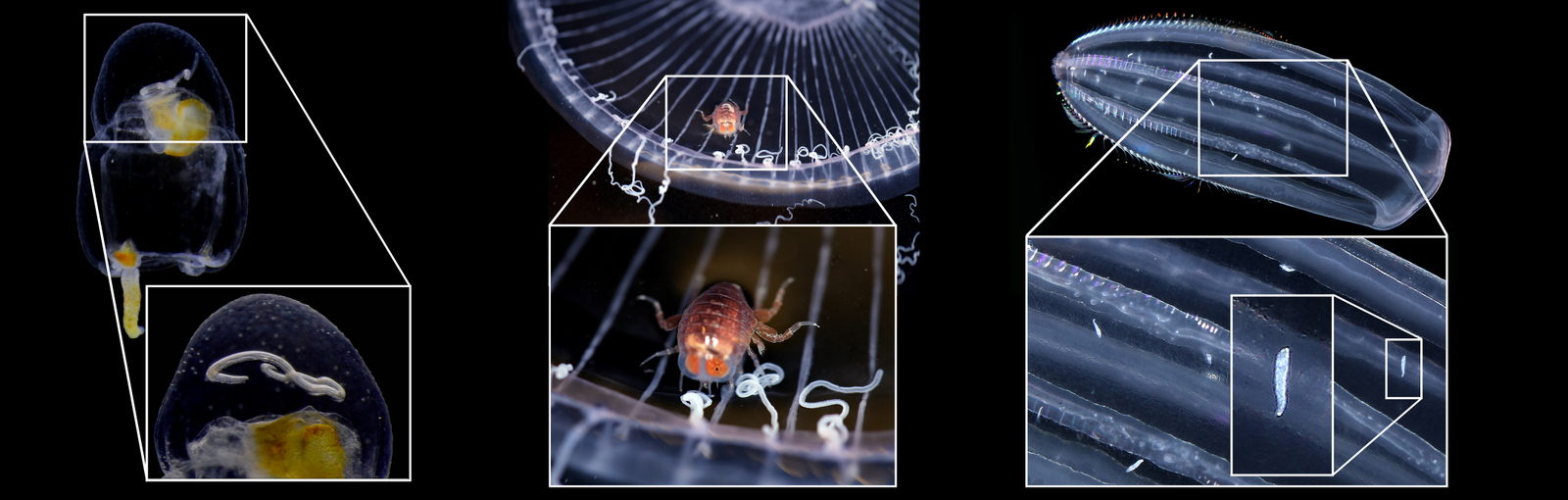
Cruises, especially on cutting-edge icebreakers crashing ice on their way to the poles, are a lot of fun, but getting deep-water samples of both jellyfish and polyps is all but easy. Consequently, even when we carefully optimize the sampling procedures, some fragile species often arrive damaged or in bad shape. This time, we collected shallow-water specimens using our own hands. In addition to (MANUAL!) net deployments at ca. 180 m depth, we intensively checked marinas, tide pools, and shallow infralittoral environments by snorkeling. It worked fantastically well for those picky jellies and polyps which tentacles/polyps get lost after being trawled or towed with a tone of hard-bodied invertebrates and slimy fish within massive nets. We took the opportunity to document them in detail using both microscopes and macro photography. During our trip, we had the visit of Stine and Linda from Artsdatabanken. They even kindly gave us a hand collecting jellyfish from the piers! Good catch!

Wide array of sampling methods, landscapes and local fauna, including a brand new wet lab (before: living room) specially made for the occasion. Stine and Linda testing our hand nets and successfully collecting some jellies. Pics: Joan Soto, Praveen Raj.
Each of us had our favorite species and top findings for the trip, and we were really excited to see alive and in good shape those species we have only examined within preserved collections, or only read about in scientific publications. These findings will definitely contribute to a better knowledge on the true diversity and distribution of these little known species, some of which have rarely been reported that far north in continental Norway.
I (Joan) stop here and leave you with some insights from the other NOAH team members that joined the trip: Marta and Praveen.
From Marta:
This was such a very interesting experience! During my work I usually study deep ecosystems, so I do not often see fresh samples of benthic hydroids, and when I do, they are often damaged by the sampling gear (e.g. bottom trawl, beam trawl…). This trip to Torsvåg has allowed me to sample the intertidal pools and marinas with my own hands, discover the great diversity that exists just below the surface, and to see multiple species in a way I never seen before.
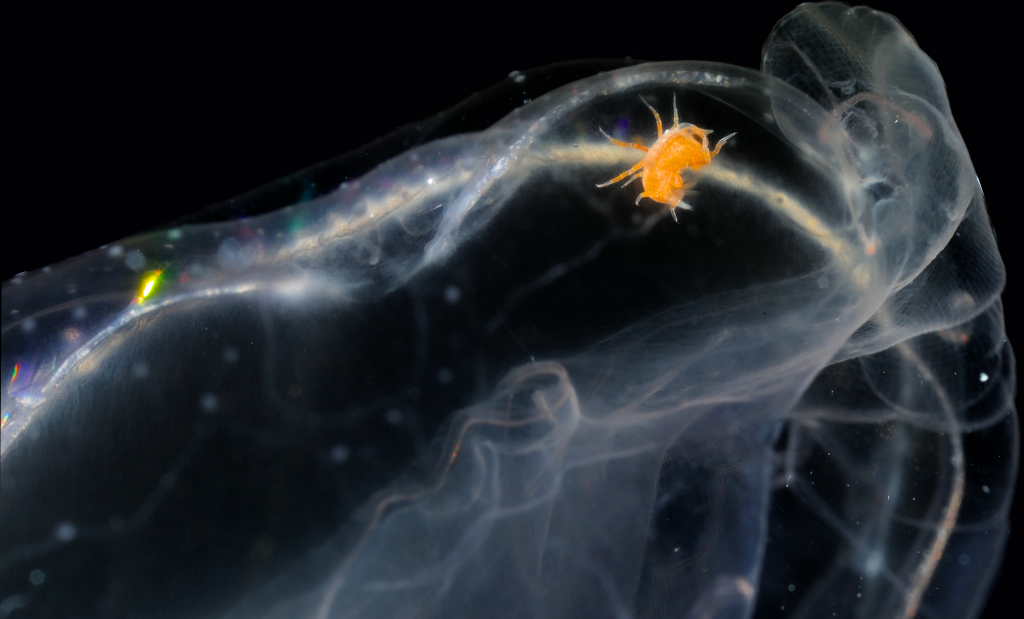
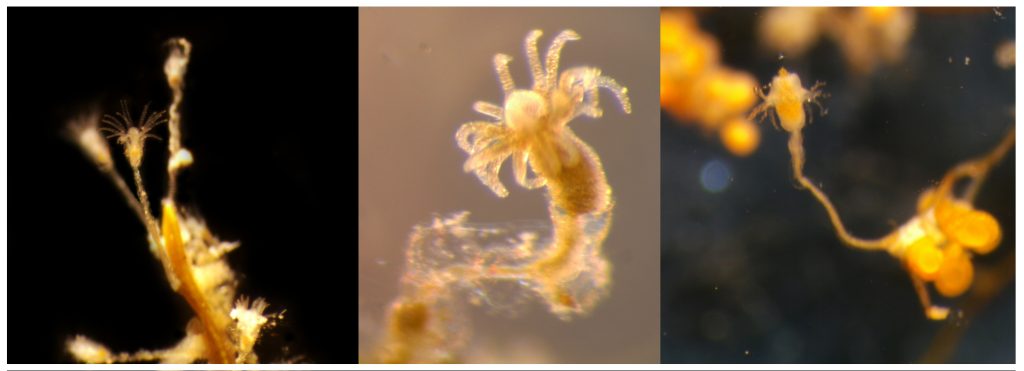
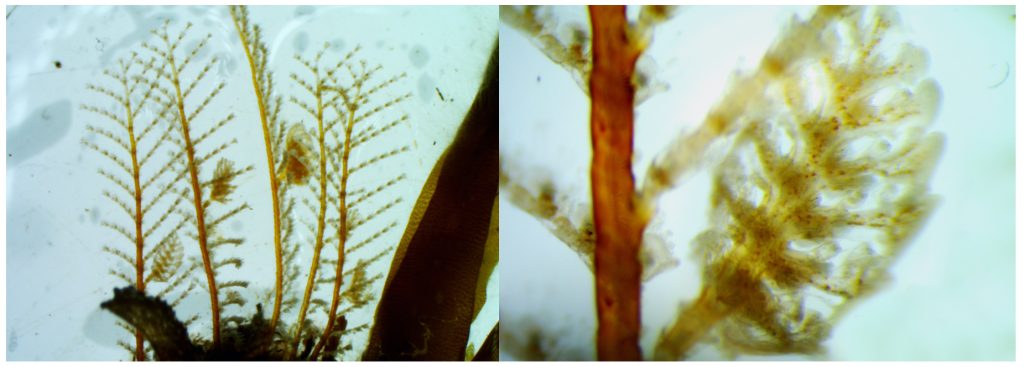
For me, the most interesting species were undoubtedly the athecate hydroids, such as the incredibly beautiful Zanclea spp. and Sarsia spp. These animals are extremely fragile due to the lack of a theca to protect the polyp. In Torsvåg, I saw athecate hydroids of extraordinary quality, studied their sexual structures, their morphology, colours, behaviour… Simply: WOW!!! I’m very much looking forward to the next NOAH trip!

During our sampling trip, Marta Gil (visiting researcher) showed a lot of excitement about the diversity in the intertidal pools and collected beautiful athecate polyps, all with reproductive structures! Pics: Joan Soto.
From Praveen:
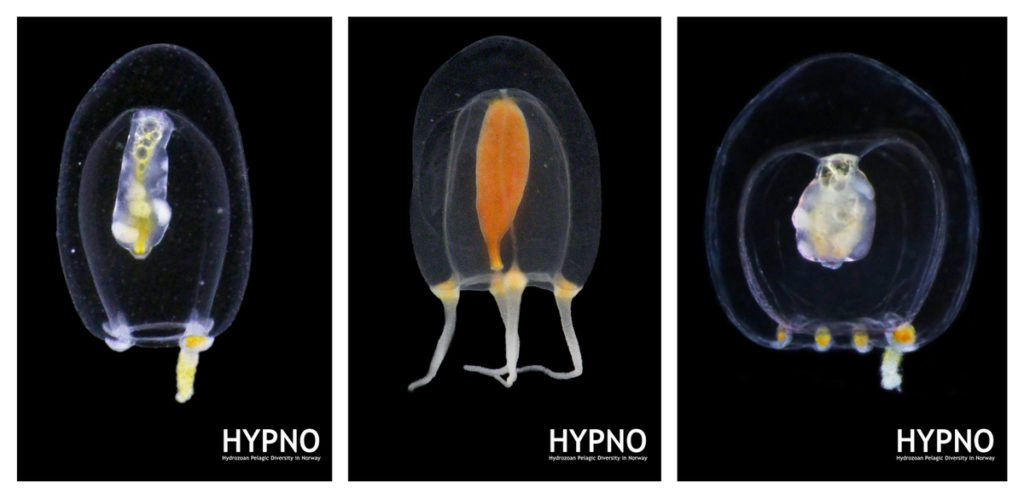
My first sampling trip after joining the Cnidaria and Ctenophora Team as a PhD student was nothing short of exhilarating. Our team, led by Joan Soto, was focused on various life stages and projects related to the phyla Cnidaria and Ctenophora. Although I had conducted similar sampling procedures in India, working with live specimens of both planktonic and benthic stages of Cnidarians in Torsvåg was a new and fascinating experience.
One of the trip’s most challenging parts was venturing into open waters with a small fishing boat to collect samples. We had to troubleshoot a bit on board, but it finally turned out to be a success. Despite not finding Dimophyes arctica, one of the target species for my PhD, I was captivated by the immense diversity of hydroids in the piers near the island, some of which might be the so-far unknown polyp stage of some known Norwegian hydromedusae. Special thanks to Luis and Marta, who taught me a lot about hydrozoan taxonomy, and Cessa and Jon for driving the boat and patiently wait for the net to be recovered.
The research was undoubtedly the focus, but the social activities and adventures we shared were equally memorable. Torsvåg itself was mesmerizing, with its high mountains and breathtaking views. We all enjoyed evening hikes and took advantage of the midnight sun, which provided a perfect balance to our intense sampling schedule. Our group dinners were another highlight of the trip for me: with colleagues from 7 different nationalities and 3 continents, we had a delightful variety of cuisines as we took turns for preparing dinner.
All in all, this sampling trip was an incredible blend of scientific discovery and personal enrichment. The stunning landscape and the camaraderie of our team made it an unforgettable experience.

Praveen Raj on his first sampling trip for his PhD. He not only captured and identified an impressive amount of jellyfish, but also had a great time hiking around Torsvåg and enjoyed the dramatic landscapes that Northern Norway can offer. Pics: Joan Soto, Praveen Raj, Lea Dober.
From Joan, Marta and Praveen.