Yesterday’s blog was about about Lucia, the mythical virgin who is celebrated with lights produced from combustion energy in candle lights. Lucia’s name is derived from the latin lux, meaning light. Similarly, Lucifer was one who was carrying light. We will not dwell with the ridiculous lore about Lucifer in old folk beliefs. Instead we will briefly look at real carriers of light from the organic world. Light can also be produced with other processes than fire and a range of organisms are particularly skilled in “letting there be light” in their environments. This is what we call bioluminescence. Bioluminescent substance can be used by potential prey animals to scare away predators. (Hover over picture with your mouse to see the gif.)
“Glow worm” beetles or “fire flies” of the families Lampyridae and Phengodidae are familiar to most people who have been out-door on a dark summer night. Studies of fire fly behavior have revealed how different species of these beetles communicate with their kinds using flash signals with variable frequency, intensity and duration. The biochemical mechanisms at work during bioluminescence are also relatively well studied in these beetle groups.
In the marine environments bioluminescence is known from many unrelated organisms and because different molecular reactions are involved in light production it is likely that bioluminescence must have evolved independently many times. A glowing sea may be experienced when massive densities of dinoflagellates are flashing their lights on the coast at night. Particularly Noctiluca scintillans, whose body is big enough to be visible with the naked eye, is frequently referred to in field guides to marine shore life. But many dinoflagellate species are involved in the bioluminescence that Norwegians call “morild”, – the “fire in the sea”. This phenomenon has also been called phosphorescence, however this is the process where light energy is absorbed by a substance and emitted on a different wave length. Special fluorescent proteins are responsible for glow-stick effects in organisms. (Incidentally the Greek light-carrier Phosphorus has been equated with the Latin Lucifer, and those of us who have seen white phosphorous in action understand what gave the name to this very reactive version of the element.) Studies of dinoflagellates have associated the light production with molecular bodies named scintillons and demonstrated that the biochemical activity in these objects in diatoms is governed by diurnal rhythms. This appears to make sense, because what is the point of flashing lights at day time? It may not be quite clear what use the diatoms have of producing light at night time either. However, it seems more obvious that there are functional advantages of bioluminescence in the depth of the ocean, where light does not penetrate. And it is below the so-called euphotic zone, from approximately 200 m on that bioluminescence is effective in different sorts of interactions among various animal groups. Deep water angler fish even use lit lures to attract prey.
Light emission in some animals is based on symbiosis with bacteria such as Aliivibrio fischeri. In other cases, the animal itself may produce the active proteins. Different biochemical systems are at work in bioluminescence and the light-producing molecules are not the same in all systems. Still, as a group of oxidizing and light emitting molecules they all go by the name of luciferin. To produce a light flash a catalyzing agent is also needed. This is provided by a group of different enzymes called luciferase. Other active components and free ions may be involved in the reaction which may be triggered in different ways, either mechanically as with a set of oars and a rowboat when dinoflagellates are near the surface water, or by some biochemical trigger. Sometimes it must happen by some neural response in the animal.
The lantern fishes are known for their photophores, – series of small organs that can produce yellow, green or blue light. Because the arrangement of photophores is different in different species, the organs are thought to play a role in communication between con-specific individuals. This may be the case for other animals as well, such as squids. It is also believed that the light organs can have a camouflaging effect by visually breaking up the silhouette of the fish, when the fish is viewed against a lighter back-ground higher up in the water column. The fish thus obtains protection from predators below by means of counter-illumination.
Several deep water animals are confusing potential predators by ejecting a luminescent substance towards the predator. Shrimps of the family Oplophoridae are particularly known to exercise this defensive technique. The luciferine in shrimps is called coelenterazine and is presumably produced in the digestive gland called haepatopancreas. When the shrimp spews the glow through the mouth, the effect is somewhat similar to the one that cephalopods use when they disappear in a cloud of ink. May we call it a “lucifer smoke-screen”?
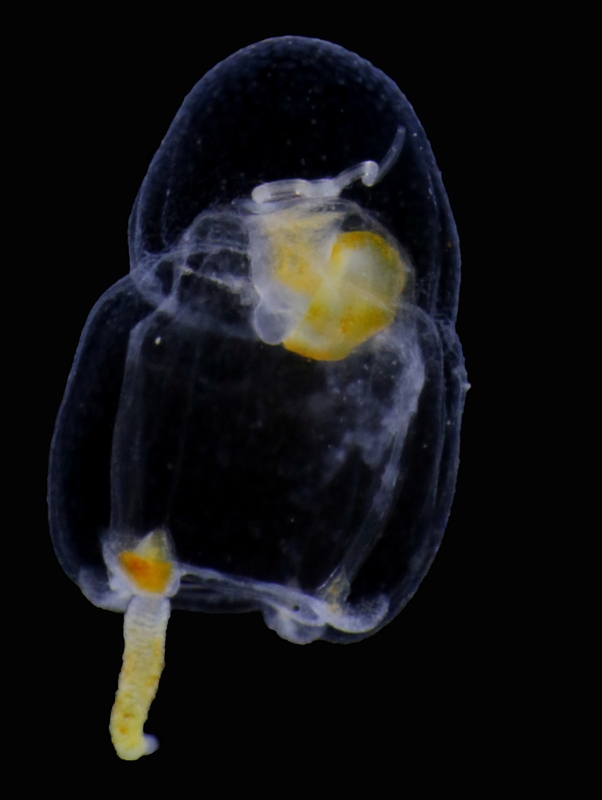
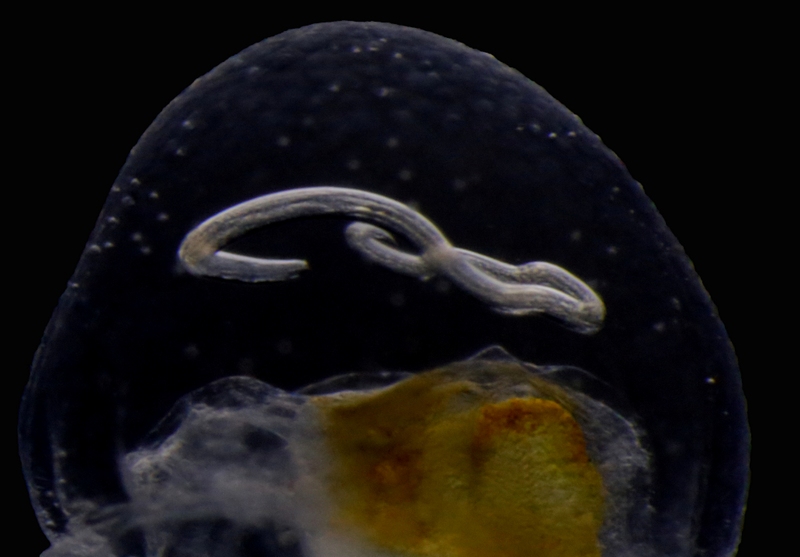
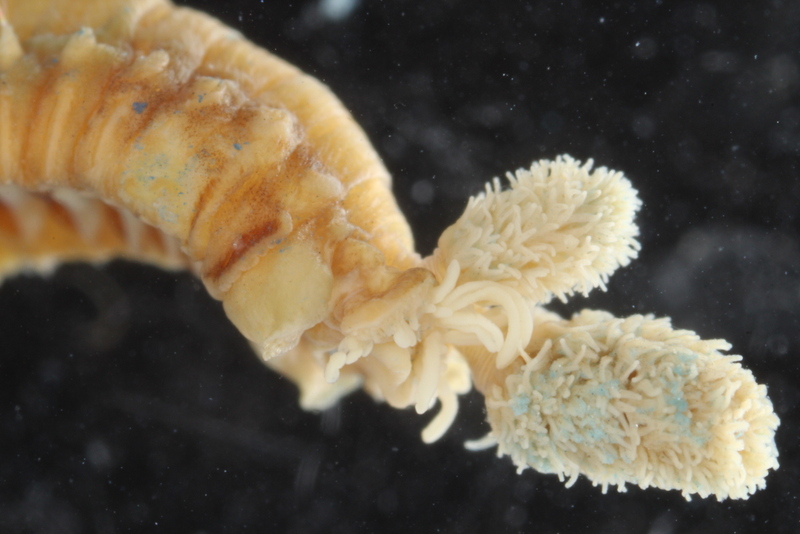
Our museum collections have a rich material of these shrimps. Several species were collected during the MAR-ECO cruises over the Mid-Atlantic Ridge. One of them was Oplophorus spinosus shown in the picture below. While all of the oplophorids appear to be able to use a “lucifer smoke”, some of the species, including O. spinosus, also have light producing organs along their sides, somewhat similar to what we see in the lantern fishes. These photophores are complicated light generating organs with lenses and reflectors. They may be able to filter the wave length, and also the intensity and direction of the emitted light. In oplophorids, such organs are only found in three of the most closely related genera of the family, according to a recent study by Wong et al. (2015). Interestingly, these animals also have two types of eye pigments. One type is shared with other oplophorids and is sensitive to the blue-green part of the light specter. The other pigment is also sensitive to the shorter wave length in UV light. Because of the special abilities of the eyes it is tempting to think that these shrimps somehow are using the photophores in communication with individuals of their species. If so, Oplophorus spinosus and similar light talk would be a perfect case for biosemiotics. “Please shrimp, tell us about the world view from your perspective!” However, it is possible that the use of the photophores is only for counter-illumination when the shrimps are performing vertical migrations in the water column.

Oplophorus spinosus – a bioluminescent mid-water shrimp carrying large eggs (Photo: David Shale, MAR-ECO).
The “signalling abilities” of bioluminescent compounds are exploited in biotechnology and cell research. Luciferase from Oplophorus has been exploited as a so-called reporter gene in visualization of cell activities and gene transcription. May be it is not too far-fetched to see the shrimps as some kind of “light-carriers”.
Papers
Inoue S, Kakoi H, Goto T. (1976) Oplophorus luciferin, Bioluminescent substance of the Decapod shrimps, Oplophorus spinosus and Heterocarpus laevigatus. J.C.S. Chem. Comm. 966:1056-1057.
(2013).Mitogenomic sequences and evidence from unique gene rearrangements corroborate evolutionary relationships of Myctophiformes (Neoteleostei). BMC Evolutionary Biology 13:111.
Shimomura O, Masugi T, Johnson FH, Hanedal Y. (1978) Properties and reaction mechanism of the bioluminescence system of the deep-sea shrimp Oplophorus gracilorostris. Biochemistry 17:994-998.
Wong JM, Pérez-Moreno JL, Chan T.-Y, Frank TM, Bracken-Grissom HD. (2015) Phylogenetic and transcriptomic analyses reveal the evolution of bioluminescence and light detection in marine deep-sea shrimps of the family Oplophoridae (Crustacea: Decapoda). Molecular Phylogenetics and Evolution 83:278–292