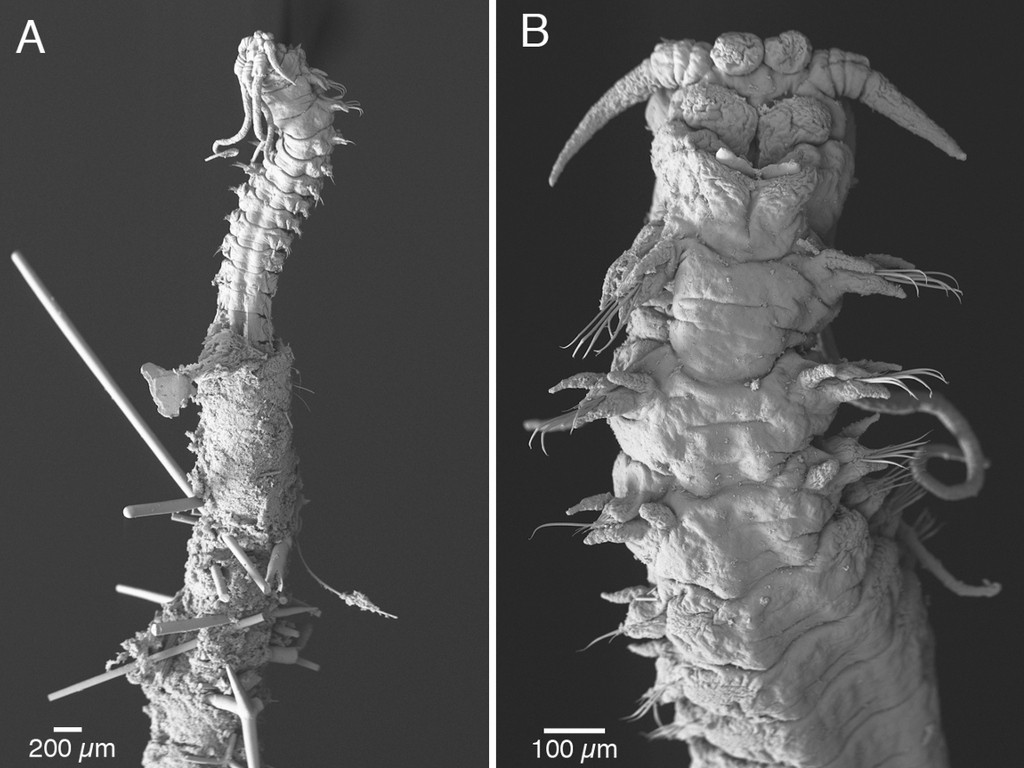
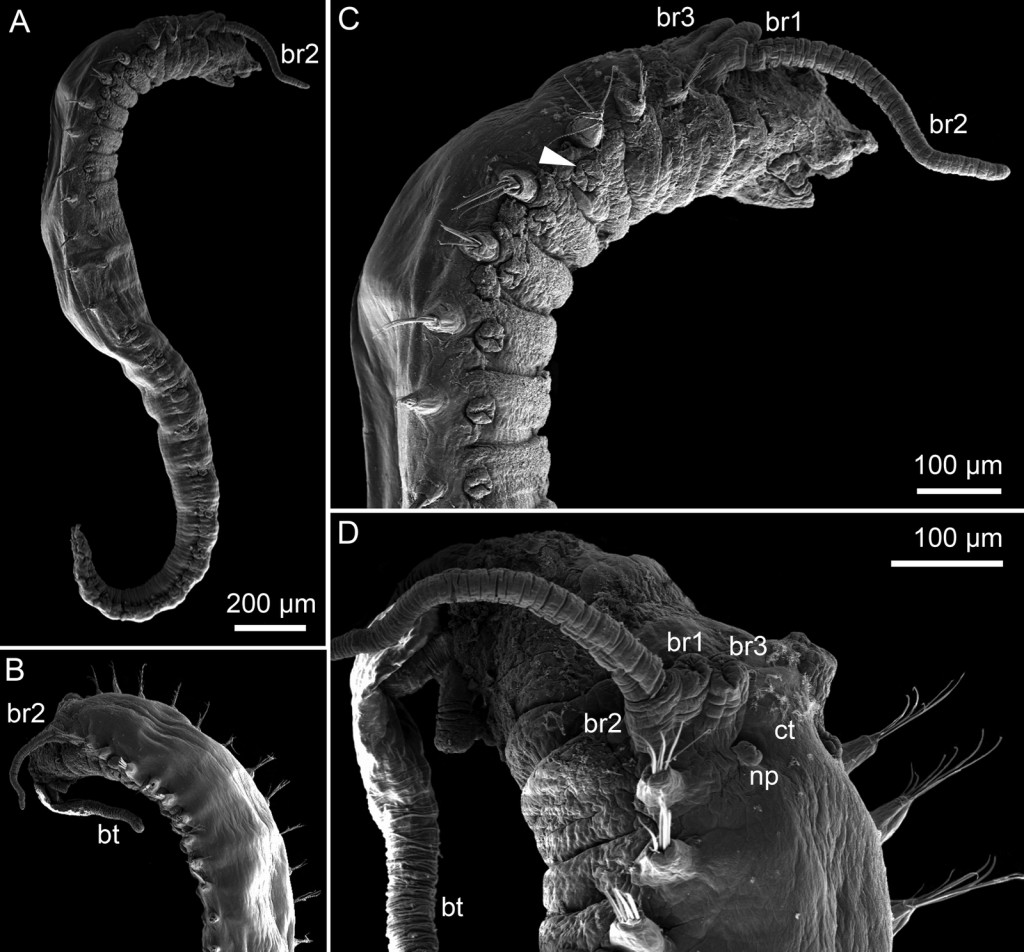
In 1939 the Swedish malacologist Nils Odhner described the nudibranch Berghia norvegica based on two specimens collected at Frøya and Stjørna in the mouth of the Trondheimsfjord.
After its original description this species has been found very few times, the first of them by Hennig Lemche a Danish malacologist who in 1958 collected a single specimen, today housed at the Natural History Museum of Bergen (ZMBN 62033). The importance of this specimen, until recently the only one apparently available in museum collections, was demonstrated by its use in a systematics review of the genus Berghia recently completed by a team of Spanish and American researchers.
The original description of Berghia norvegica is fairly detailed, but was based on preserved specimens and therefore the colouration of this species remained elusive until very recently. For over half a century nothing was known about the colouration of this beautiful and unique animal and is only in 2011 and subsequent years that Berghia norvegica is finally rediscovered by divers and researchers participating in the NudiSafaris organized at Gulen in Sogn og Fjordane just north of Bergen.
These recent discoveries revealed the extreme beauty of this delicate animal and generated the first live images of this endemic and emblematic species of the Norwegian fauna, which we here illustrate with a photograph taken at Gulen on March, the 15th of 2014 at 38 m deep and kindly made available by Kåre Telnes author of the website “The Marine Fauna and Flora of Norway”.
Suggested reading:
Carmona, L., Pola, M., Gosliner, T. M. & Cervera, J. L. 2014. The Atlantic-Mediterranean genus Berghia Trinchese, 1877 (Nudibranchia: Aeolidiidae): taxonomic review and phylogenetic analysis. Journal of Molluscan Studies, 80: 482–498.
Evertsen, J. & Bakken, T. 2013. Diversity of Norwegian sea slugs (Nudibranchia): new species to Norwegian coastal waters and new data on distribution of rare species. Fauna Norvegica, 32: 45–52.
Odhner, N.H. 1939. Opisthobranchiate Mollusca from the western and northern coasts of Norway. Det Kongelige Norske Videnskabernes Selskabs Skrifter, 1: 1–93.