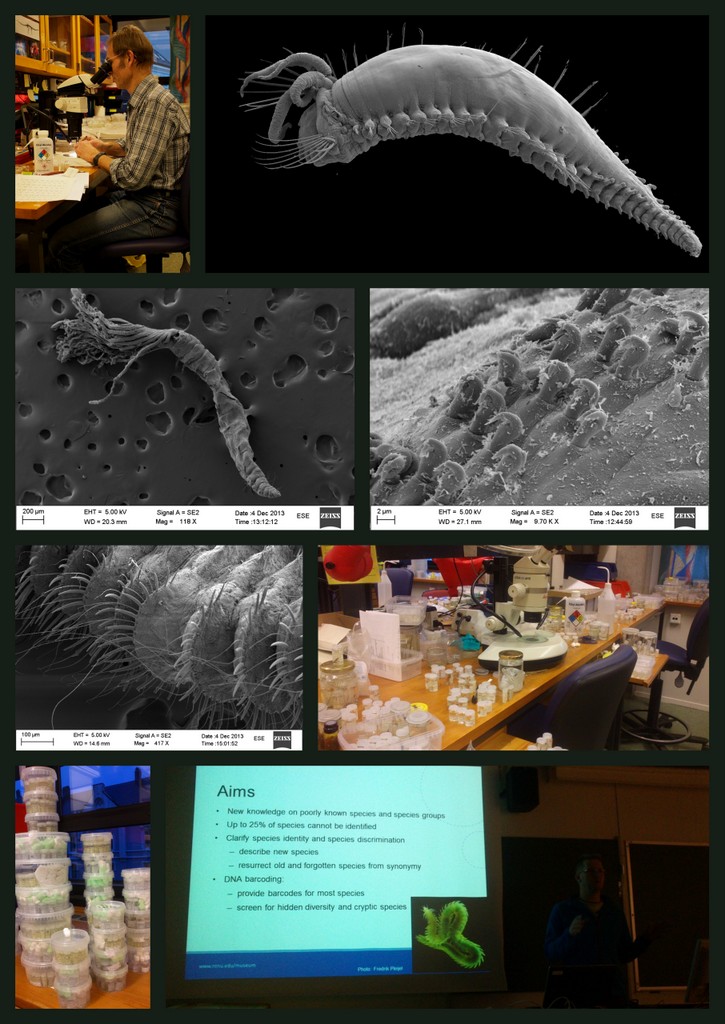
As usual, we use a variety of methods to work with our animals – these include use of stereo microscope, “ordinary” microscope, and electron scanning microscope (SEM). Below are some pictures of work in progress during today.
Category Archives: Current projects
PolyNor Workshop on the MAREANO material
This week our lab is teeming with activity as twelve researchers goes to work on our polychaete material, focusing mainly on that which has been collected by the MAREANO program.
As mentioned previously, the material collected by MAREANO gets split into size based fractions, which then receive different treatments. As far as the polychaetes go, MAREANO carries out routine identification on what is collected by grab (1 mm) and beam trawl (5 mm), all of which has been fixed in formalin (until this year, where it was begun fixing half of the beam trawl in ethanol). Thus we already have some idea of what to expect to find in the material.
At the Invertebrate Collections we have processed a lot of bulk samples from the fractions that MAREANO does not utilize, and lately we have especially focused on the Ethanol fixed material, as this can be used for genetic work.
This workshop is part of the Polychaete diversity in the Norwegian sea (PolyNor) project. This project aim to explore the diversity of polychaetes in the Nordic seas;
The Norwegian Sea holds a diverse fauna of polychaete worms, more diverse than previously anticipated. Recent work has discovered several new species and species described in the old literature but not seen since their description, has been rediscovered. Material from new samples will be targeted to discover the true diversity of polychaetes in the Norwegian Sea.
During the workshop we will work on some of the groups with especially tricky taxonomy, prioritizing the identifying of specimens fixed in ethanol, and select individuals that are especially suited for genetic work. And of course we will also discuss current topics, find and reconnect with collaborators, drink an unholy amount of coffee, and learn some new things!
Home, sweet home
I came across these two bristle worms from the genus Nothria whilst sorting out the animals from a sample collected in the Barents sea by the MAREANO project, and wanted to show you how differently they’ve approached the choice of building materials for their tubes. They build the tube around their bodies to protect themselves from predators. Now, a Nothria outside its “house”, or tube, looks like this (scale bar is 2 mm) :
The same animal inside its tube looked like this:
And then there was this one, who had made a more select choice of building materials:
Fall cruise with MAREANO
I’m onboard the research vessel “G.O. Sars”, participating in the last MAREANO cruise of the year. We’re currently on our way back out to the sampling sites after seeking refuge in a fjord from the storm yesterday.

Sampling areas. The yellow area is finished, the brown ones are work in progress. From mareano.no
The area we’re working on is outside Møre & Romsdal, currently we’re on our way to a set of video stations whilst we wait for the sea swell to die down (it’s quite the rollercoaster here at the moment!). We have two-three full stations remaining, hopefully we’ll be able to finish those as well before the cruise ends this Friday.
“Full station” means that we in addition to videoing the sea floor for a 700m long transect with our remotely controlled video rig, the Campod 2, also collect physical samples.
This is done using a variety of gears, which collectively gives us a extensive insight in the properties of the area we’re working on. On board we have a team of biologists, geologists and a chemist. The geologists and chemist are after sediment cores, which provide a window back in time for analyses of the physical and chemical parameters of the sea floor, including the accumulation of pollution. How far back a core extends will depend on the sedimentation rate, and on how long the core we manage to extract is.
For collecting animals, we are using three main gears: the epibenthic RP-sled, the beam trawl, and the grab. These collect different parts of the fauna, and (together with the video) gives us a fair understanding of the species diversity and composition.

The grab (a van veen) collects a quantifiable amount of animals exceeding 1 mm in size living in the sediment. We collect two grabs at each full station.

A typical grab sample. We carefully rinse the mud through a 1 mm sieve, collecting the animals within it.

RP sled (left) and the beam trawl. The sled collects the small animals living just above and in the upper layer of sediment. The beam trawl collects the macro- and megafauna living above and within the top layer of the bottom.

Fulmars and gulls are following us, hoping we’ll give up on the small animals and start catching fish for them
Now we’ve arrived at the next station, so I’d better get going!
Summertime..
..is conference time!
Most of our department are currently either attending The World Congress of Malacology, or are busy preparing for The 11th International Polychaete Conference.
The blog is therefore entering a bit of a summer holiday, though there may be some glipses of conference life coming.
Sponges!
Hunting Slugs in The Bahamas
Recent research has showed that something is going on in the Bahamas! Even when specimens from these islands look pretty much alike its “con-specifics” from other parts of the Caribbean region, they show considerable genetic divergence. Likely ecological and/or oceanographic processes are limiting gene-flow between the Bahamas and nearby islands accelerating the rate of speciation.
At the University Museum we want to understand the “entrails” of these processes and therefore we headed to sunny Bahamas for a two-weeks fieldtrip in Eulethera I. Seventeen specimens of opisthobranchs gastropods have been collected and two populations of our model-species, the cephalaspidean Bulla occidentalis, were found inside closed ponds lined by mangroves and limestone. These ponds are very special habitats completely enclosed and only communicating with the ocean by submarine outlets or through the porosity of the limestone rock and can be considered “islands inside islands”.
DNA will be soon extracted from specimens of the two populations of Bulla occidentalis and compared with that of other populations throughout the tropical West Atlantic from Brazil to Bermuda. This will help understanding processes of historical biogeography and speciation in the highly complex Caribbean region.
Further work on West African biodiversity
In addition to the crabs (Brachyura) discussed in the previous post, we are also focusing on animal groups such as the brittle stars (Ophiuroidea) and bristle worms (Polychaeta).
Currently we are preparing samples for genetic barcoding though the BOLD system.
Here are a couple of photographs of the animals that have been through the mill of identification – photo documentation – tissue sampling this week.

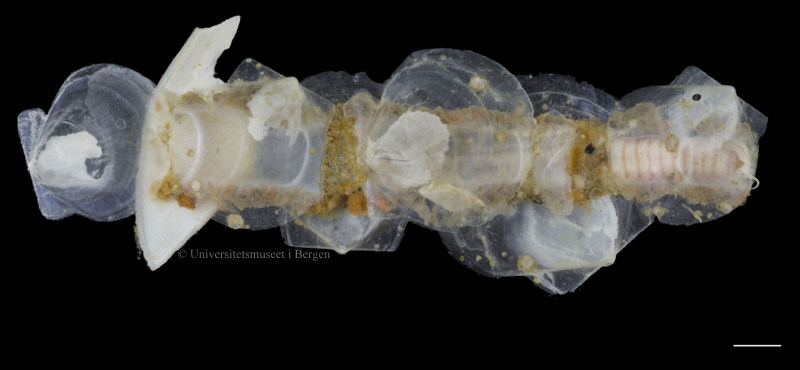
A bristle worm from the family Maldanidae, partially encased in the tube that the animal dwells in (scale bar is 0.5 cm)

Another and rather different looking bristle worm, this time from the family Onuphidae. Scale bar is 0.5 cm
Focus on West African crabs (Brachyura)
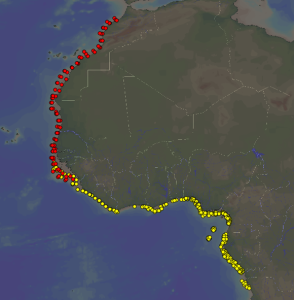
R/V Dr Fridtjof Nansen sampling stations for which benthic samples have been deposited in the Invertebrate Collections of Bergen. Red dots: the Canary Current Large Marine Ecosystem (CCLME). Yellow dots: the Guinea Current Large Marine Ecosystem (GCLME)
Since 2005 the research vessel R/V Dr Fridtjof Nansen has been sampling benthic invertebrates on the continental shelf of the large marine ecosystems (GCLME and CCLME) of West Africa. A large bulk of the material is kept in our collection and is being processed for taxonomic and other studies by several workers.
These days we are particularly focusing on the true crabs (Brachyura) and are preparing specimens for DNA barcoding with the BOLD system. This work will produce open access data (genetics, morphology, distribution) to enhance a broader knowledge about Atlantic marine biodiversity. The project is financially supported by JRS Biodiversity Foundation.

Cronius ruber (Lamarck, 1818) caught off Guinea at 35 m depth in May 2012. (Identification E.Willassen)

A small assembly of crabs photographed and prepared for DNA barcoding. Some specimens have still kept some colors despite being preserved in ethanol
Bioskag Workshop
We began the year with arranging two workshops in January. The first of these was based on the extensive sampling done in the Skagerrak strait in recent years (2006 – today). The aim was to increase the rather poor knowledge of the invertebrate fauna of the region (link is in Norwegian).
The focal group of this workshop was the Polychaetes (bristle worms), and through the effort of the ten attendants, a lot of material was examined. You can read more about this at the blog of the Norwegian Polychaete Forum.
We are currently processing the findings from the workshop, including documenting specimens through photography and drawing. One of the tools we employ for doing this is the scanning electron microscope (SEM), which enables us to make high resolution images at a very high magnification.