We’re spending the first two weeks of July out on our marine field station, running a workshop with participants from nine different countries on material from Western Africa. You can read more about this on our project blog: Marine Invertebrates of Western Africa
Author Archives: katrine
Fluid Preservation Course
Curating a natural history collection comes with many challenges; how do you “freeze” the specimen in such a state that another taxonomist can request to examine it in 10 (20, 30, 50, 100…) years from now, and expect to find the same characters (the traits that are used for determining which species one is looking at) as the one who originally described or determined the specimen?
Which fluid should then be used as a preservative? Here we nee to take into consideration such features as potential harmfulness, fire hazards, longviety, the possible effects on histology and DNA, resistance to pests, effects on the container it is kept in, etc. etc. And how should the samples be stored? How do you rescue objects that have been damaged?
I spent most of last week attending a course in methods for fixing and preserving natural history specimens in fluid; The «Fluid Preservation Course» was given by Simon Moore at The Horniman Museum in London.
Some of the topics covered included
- different methods and chemicals for fixating and preserving specimens,
- how to work with glass (cutting, drilling and grinding), especially how to make lids for jars of various sizes,
- how to make and repair objects for display,
- how to salvage specimens that have been damaged due to dessication, fungi or other perils,
- avaliable chemicals and their properties,
- how to determine which chemicals the animals are stored in

Making a display part 1: One lizard in a bag. Extract from bag, figure out which preservative has been used, transfer to suitable new preservative.

Making a display part 2b: Stitch monofilament in to lizard to mount it on a custom made piece of glass in the jar (it looks rather brutal, doesn’t it?)
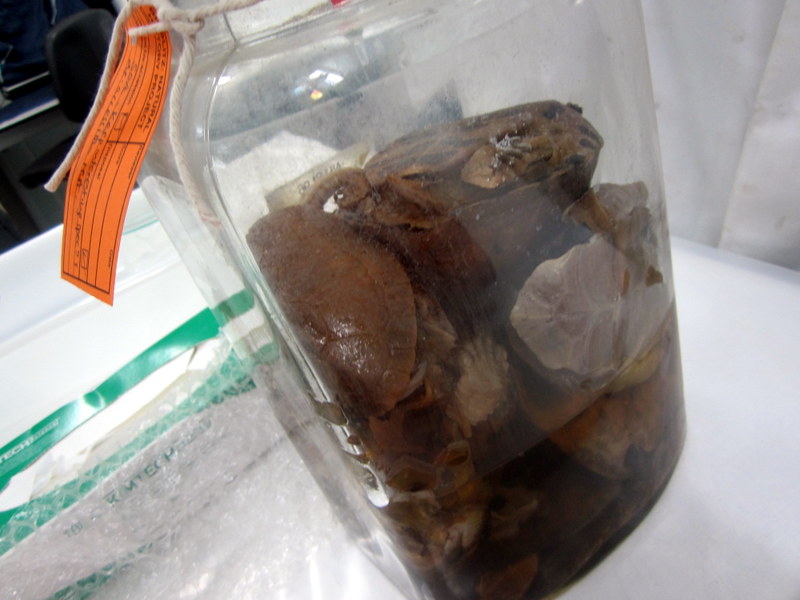
A jar stuffed full of turtles and tortoises in really bad condition – we tried to salvage as much as possible, especially one specimen that was of a species they didn’t have in the museum collection.

The spiders have been mounted on monofilament, the missing legs have been re-attached using class needles and colloidin as glue, the jar has been replaced, and they are now stored in 80% alcohol.
Sponges!
Further work on West African biodiversity
In addition to the crabs (Brachyura) discussed in the previous post, we are also focusing on animal groups such as the brittle stars (Ophiuroidea) and bristle worms (Polychaeta).
Currently we are preparing samples for genetic barcoding though the BOLD system.
Here are a couple of photographs of the animals that have been through the mill of identification – photo documentation – tissue sampling this week.

A bristle worm from the family Maldanidae, partially encased in the tube that the animal dwells in (scale bar is 0.5 cm)

Another and rather different looking bristle worm, this time from the family Onuphidae. Scale bar is 0.5 cm
Launching PolyNor
Meet catalogued specimen # 90.000
We recently rounded 90.000 specimens in our scientific collections, and since # 90 000 is such a good representative of what we are currently working on, we’re presenting it here.
This is a Cumacea, or hooded shrimp. It was collected by the MAREANO project. It was then identified by a taxonomist on a workshop arranged by the Department of Biology (UoB), the University Museum and MAREANO. It was then implemented in the Museum’s scientific collection, and chosen to be used as a DNA voucher for the NorBOLproject (Norwegian Barcode of Life). Here it will be part of a comprehensive library of standardized DNA sequences (barcodes) which will serve as a reference resource for the research and management of biodiversity in Norway.


















